Vinyl chloride is an organochloride with the formula h 2 c chcl that is also called vinyl chloride monomer vcm or chloroethene this colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc.
A polymer of vinyl chloride example.
Polyvinyl acetate pvac many vinylidene and vinylene compounds polymerize in the same manner.
It is highly susceptible to uv and high temperature degradation and stabilizers see stabilizers in the polymer additives section are commonly added.
Plasticized pvc polyvinyl chloride so boots and coats and stuff like that or unplasticized pvc for sidings and windows etc.
Vinyl chloride ch2 chcl is most often obtained by reacting ethylene with oxygen and hydrogen chloride over a copper catalyst.
Two main commercail available polmers.
The reaction vessel is.
Polymers at the super microscopic level look like great bunches of chains or gobs of spaghetti.
Vinyl chloride may be represented by the structural formula.
Polyvinyl chloride pvc vinyl fluoride.
Major industrial polymers major industrial polymers polyvinyl chloride pvc.
A vinyl chloride based polymer was obtained by the same method described in example 1 except for performing a reaction while maintaining the polymerization temperature at 47 c.
A vinyl chloride based polymer was obtained by the same method described in example 3 except for.
About 13 billion kilograms are produced annually.
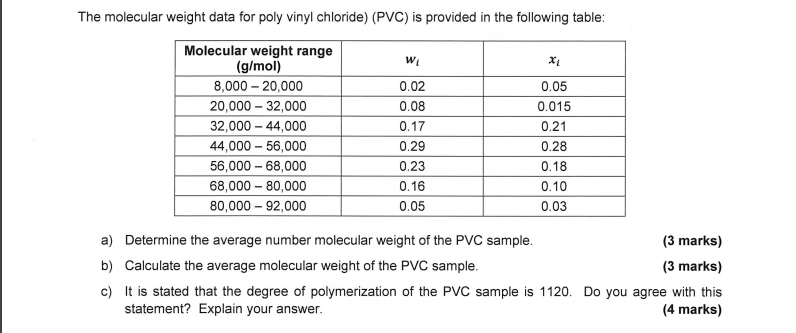
Poly vinyl chloride is prepared by polymerisation of monomer vinyl chloride about 80 percent of polymerisation includes suspension polymerisation 12 emulsion polymerisation and 8 bulk polymerisation.
And adding 0 005 parts by weight of eugenol.
Poly vinyl chloride is often highly plasticized to improve rheology for melt processing.
What is a polymer of vinyl chloride.
Vcm and water are introduced into the reactor and a polymerization initiator along with other additives.
They are made by chemically linking together many similar smaller units known as monomers.
5 18 60 61 these polymers have been used extensively in the past for corrosion protective systems but they often need large amounts of strong solvents.
Vinyl chloride c 2 h 3 cl is the monomer link in the polyvinyl chloride chain.
Those polymers are analogously referred to as polyvinylidenes and polyvinylenes reflecting the monomeric precursors.
An example would be useful.
Polyvinyl fluoride pvf vinyl acetate.
Second only to pe in production and consumption pvc is manufactured by bulk solution suspension and emulsion polymerization of vinyl chloride monomer using free radical initiators.
Vinyl polymers is a name often used to identify polymers based on the vinyl group nch ch 2 but really has been used to identify polymers and copolymers of vinyl chloride.






.jpg)